William L. Neumann, PhD
Professor of Pharmaceutical Sciences
Phone: 618-650-5088
Fax: 618-650-5145
E-Mail: wneuman@siue.edu
Organic/Medicinal Chemistry and Chemical Biology
B.S., 1983, University of Missouri Columbia
Ph.D., 1987, University of Missouri St. Louis
Biography
Dr. Neumann received a BS in chemistry from the University of Missouri at Columbia in 1983 and a PhD in 1987 from UM-St. Louis. Before joining the SIUE School of Pharmacy, he spent the majority of his career as a group leader in synthetic organic and medicinal chemistry at Monsanto Corporate Research, Pharmacia, Pfizer and Mallinckrodt. Dr. Neumann’s research interests include structure-based drug discovery, the development of new synthetic methods for applications to parallel medicinal chemistry, the development of protein nitration inhibitors and the design, synthesis and photophysical optimization of fluorescent probes for optical imaging.
Research and Publications
Neumann Group Research Interests
Parallel-Friendly Catalysis. We have a long standing interest in developing selective cross-coupling methods for the parallel decoration of aryl and heteroaryl scaffolds. For heteroaryl cross coupling strategies we have developed “Switchable-Catalysis” methods which employ orthogonal reaction chemistries. By the appropriate choice of reaction conditions (base versus no base for example) we are able to switch between Suzuki coupling of an aryl boronic acid with a heteroaryl bromide and Liebeskind-Srogl coupling of an aryl boronic acid and a heteroaryl methylsulfide. We are also interested in transition metal catalyzed routes to functional groups which are difficult to prepare under standard conditions. To this end we have developed a new reagent and strategy for the synthesis of protected benzamidines using a cross-coupling approach.
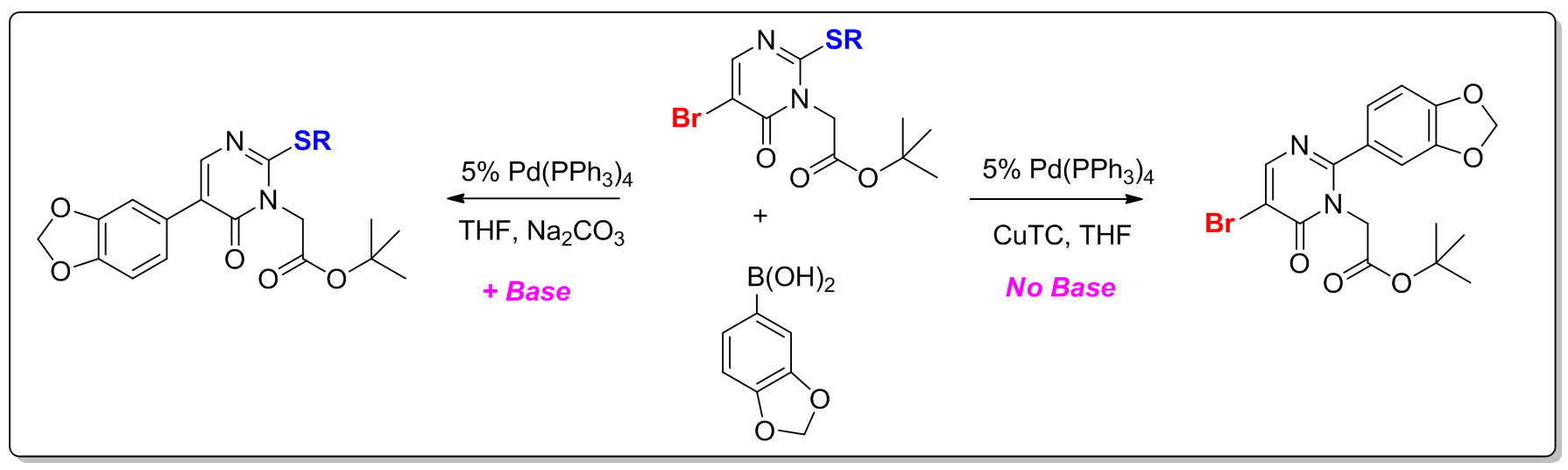
“Switchable Catalysis: Modular Synthesis of Functionalized Pyrimidinones via Selective Sulfide and Halide Cross-Coupling Chemistry” Org. Lett, 2003, 5, 4349.
“A New Catalytic Cross Coupling Approach for the Synthesis of Protected Aryl and Heteroaryl Amidines” Org. Lett. 2002, 4(6), 983-985.
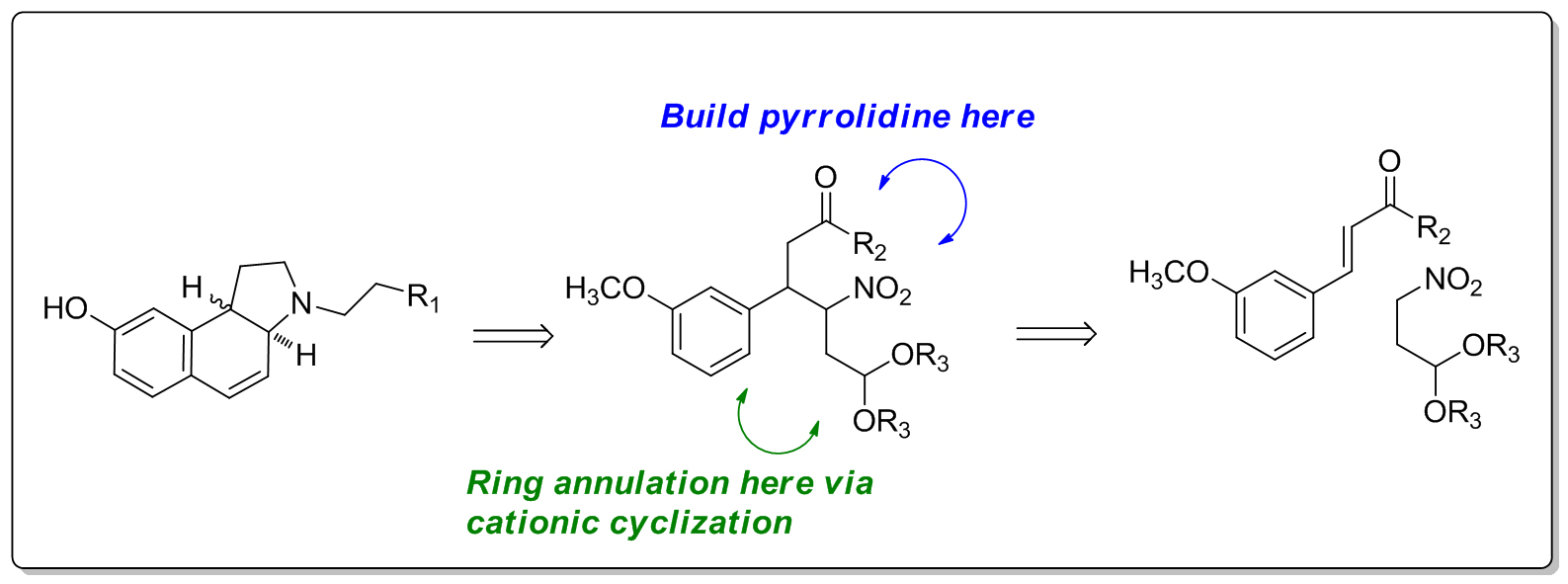
Synthesis of Heterocyclic Derivatives for Drug Discovery. We are currently developing new synthetic routes to pyrrolidine-based heterocyclic systems as scaffold for drug discovery. Our main emphasis is on nitroalkane-cinnamate conjugate addition chemistry and Pd-catalyzed carboamination cascades for the synthesis of benzindole analogues. The resulting compounds are being surveyed for dopaminergic and sigma receptor activity.
Metalloporphyrin and Dipyrromethene-Based Protein Nitration Inhibitors. In this area we have developed a series of metal-charge-shielded manganese(III)porphyrin and dipyrromethene derivatives which function as potent orally active peroxynitrite decomposition catalysts. Peroxynitrite is the short-lived and highly toxic reaction product of superoxide and nitric oxide. It is overproduced in vivo under conditions associated with a variety of neurodegenerative and metabolic disease states. The uncatalyzed decomposition manifold of elevated levels of peroxynitrite leads to protein tyrosine nitration in vivo. Protein tyrosine nitration is almost always related to loss of protein function or gain of an unwanted function. Our catalysts destroy peroxynitrite in vivo and are thus effective inhibitors of protein nitration. Our collaborators have demonstrated beneficial effects of catalysts from the SR class in models of inflammatory pain, chemotherapy-induced peripheral neuropathy, opioid analgesic tolerance and obesity-driven diabetes.
“Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance” Pain 2013, 154(7), 978-986.
“Targeting the Overproduction of Peroxynitrite for the Prevention and Reversal of Paclitaxel-induced peripheral neuropathy” J. Neuroscience 2012, 32, 6149-6160.
“Manganese(III) Complexes of Bis-Hydroxyphenyldipyrromethenes are Potent Orally Active Peroxynitrite Scavengers” J. Am. Chem. Soc. 2011, 133, 4200-4203.
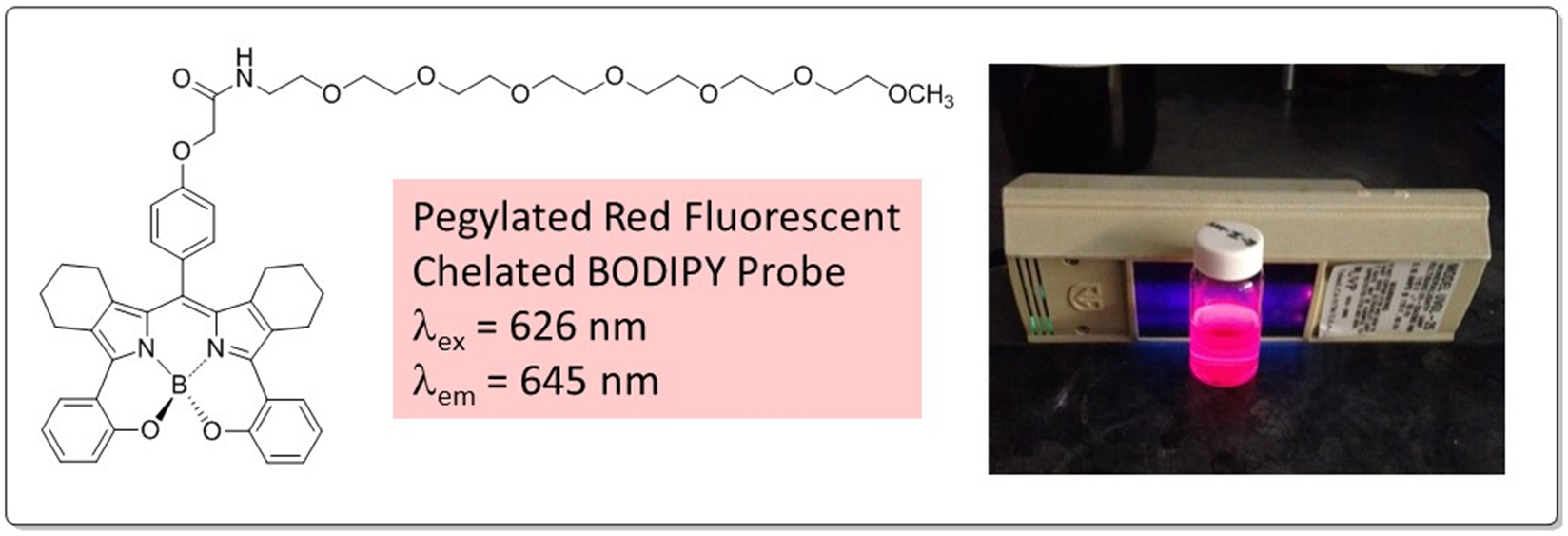
Optical Imaging, Tissue Monitoring, and Phototherapy Agents. We are interested in functional dye molecules which fluoresce in the visible to near infrared regions of the electromagnetic spectrum. Thus, our group is developing synthetic methods to construct new functionalized BODIPY and heterocyclic fluorescent molecules which are useful for cell-based and potentially in vivo imaging. We are particularly interested in dyes which have very large Stokes Shifts or can become “instantaneously activated” by a relevant biological stimulus. We are also interested in nanoparticle applications of fluorescent dyes.